Unveiling the Building Blocks of Matter: A Deep Dive into the Atomic Structure
Related Articles: Unveiling the Building Blocks of Matter: A Deep Dive into the Atomic Structure
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Unveiling the Building Blocks of Matter: A Deep Dive into the Atomic Structure. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Unveiling the Building Blocks of Matter: A Deep Dive into the Atomic Structure
- 2 Introduction
- 3 Unveiling the Building Blocks of Matter: A Deep Dive into the Atomic Structure
- 3.1 Delving into the Atomic Structure
- 3.2 The Importance of Atomic Structure
- 3.3 FAQs on Atomic Structure
- 3.4 Tips for Understanding Atomic Structure
- 3.5 Conclusion
- 4 Closure
Unveiling the Building Blocks of Matter: A Deep Dive into the Atomic Structure

The universe, in all its vastness and complexity, is fundamentally composed of tiny, indivisible particles known as atoms. These minuscule entities, far too small to be seen with the naked eye, are the building blocks of everything we experience, from the air we breathe to the stars that illuminate the night sky. Understanding the makeup of an atom is crucial for comprehending the nature of matter, the forces that govern the universe, and the fundamental laws that shape our reality.
Delving into the Atomic Structure
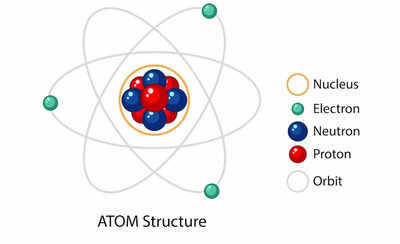
The atom, in its simplest form, can be visualized as a miniature solar system, with a dense, positively charged nucleus at its center, surrounded by a cloud of negatively charged electrons orbiting in specific energy levels.
1. The Nucleus: The Heart of the Atom
The nucleus, the atom’s central core, houses the majority of its mass and contains two types of subatomic particles: protons and neutrons.
- Protons: These particles carry a positive electrical charge and are responsible for an atom’s atomic number. The atomic number defines an element’s identity, distinguishing it from all others. For instance, all carbon atoms have six protons, while all oxygen atoms have eight.
- Neutrons: These particles carry no charge and are crucial for stabilizing the nucleus. The number of neutrons in an atom can vary, leading to the existence of isotopes, which are atoms of the same element with different numbers of neutrons.
2. The Electron Cloud: A Realm of Energy Levels
Surrounding the nucleus is a cloud of electrons, negatively charged particles that orbit the nucleus in specific energy levels or shells. These shells are not physical orbits, but rather represent regions of space where electrons are most likely to be found.
- Energy Levels: Electrons occupy different energy levels, each with a distinct amount of energy. Electrons in lower energy levels are closer to the nucleus and are more tightly bound, while those in higher energy levels are further away and less tightly bound.
- Electron Configuration: The arrangement of electrons in energy levels is known as the electron configuration. This configuration determines an atom’s chemical properties and how it interacts with other atoms to form molecules.
3. The Quantum Mechanical Model: A More Accurate Picture
While the solar system model offers a helpful visualization, it is an oversimplification. The actual behavior of electrons is governed by the principles of quantum mechanics, which describes the wave-like nature of particles at the atomic level.
- Orbitals: Instead of orbits, electrons occupy regions of space called orbitals. These orbitals are not well-defined paths but rather probability distributions, indicating the likelihood of finding an electron in a particular region.
- Quantum Numbers: Each electron within an atom is characterized by a set of four quantum numbers, which describe its energy level, shape, orientation in space, and spin.
The Importance of Atomic Structure
Understanding the atomic structure is fundamental to several scientific disciplines and has far-reaching implications for our understanding of the world around us.
1. Chemistry: The Foundation of Matter
The atomic structure forms the basis of chemistry, the study of matter and its properties. The arrangement of electrons in an atom determines its reactivity, its ability to form bonds with other atoms, and the properties of the resulting molecules.
- Chemical Bonding: The interaction between atoms to form molecules is governed by the sharing or transfer of electrons. This process, known as chemical bonding, leads to the formation of new substances with unique properties.
- Chemical Reactions: Chemical reactions involve the breaking and formation of chemical bonds, resulting in the transformation of matter. Understanding atomic structure is crucial for predicting and controlling these reactions.
2. Physics: Exploring the Universe
Atomic structure is also central to our understanding of physics, the study of matter, energy, and their interactions.
- Nuclear Physics: The study of the nucleus and its properties, including nuclear reactions like fission and fusion, relies heavily on knowledge of the atomic structure. These reactions are responsible for the energy released in nuclear weapons and power plants.
- Astrophysics: The study of celestial objects and phenomena, such as stars, planets, and galaxies, relies on an understanding of atomic structure. Atomic spectra, unique patterns of light emitted by atoms, are used to analyze the composition and temperature of distant stars and galaxies.
3. Material Science: Engineering the Future
Atomic structure plays a critical role in material science, the study of the properties of materials and their applications.
- Material Properties: The arrangement of atoms in a material determines its mechanical, electrical, and optical properties. Understanding atomic structure allows scientists to tailor materials for specific applications, such as developing stronger, lighter, or more conductive materials.
- Nanotechnology: The manipulation of matter at the nanoscale, involving individual atoms and molecules, is revolutionizing fields like medicine, electronics, and energy.
FAQs on Atomic Structure
1. What is the smallest unit of an element?
The smallest unit of an element is an atom. An atom is the fundamental building block of all matter, and it cannot be further broken down by chemical means.
2. What are the differences between atoms and molecules?
Atoms are the individual building blocks of elements, while molecules are formed when two or more atoms bond together. For instance, a water molecule (H2O) is formed by the bonding of two hydrogen atoms and one oxygen atom.
3. How are atoms different from each other?
Atoms of different elements differ in the number of protons they contain. This number, known as the atomic number, defines the element’s identity. For example, carbon atoms have six protons, while oxygen atoms have eight.
4. What is the relationship between atomic structure and the periodic table?
The periodic table organizes elements based on their atomic structure, particularly their electron configuration. Elements with similar electron configurations share similar chemical properties, explaining the periodic trends observed in the table.
5. How does atomic structure affect the properties of materials?
The arrangement of atoms in a material determines its properties. For instance, the strong bonds between carbon atoms in diamond give it exceptional hardness, while the weak bonds between atoms in graphite make it soft and slippery.
6. What are isotopes, and how do they differ from each other?
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This difference in neutron count leads to variations in their mass and some physical properties.
7. What is the role of quantum mechanics in understanding atomic structure?
Quantum mechanics provides a more accurate description of electron behavior than the classical solar system model. It explains the wave-like nature of electrons and their probability distributions within orbitals.
8. What are some applications of atomic structure in technology?
Atomic structure knowledge is crucial in various technologies, including the development of new materials, the design of electronic devices, and the development of advanced imaging techniques.
Tips for Understanding Atomic Structure
- Visualize the Atom: Use models and diagrams to visualize the atom’s structure, including the nucleus and electron cloud.
- Focus on Key Concepts: Concentrate on key concepts like atomic number, mass number, electron configuration, and chemical bonding.
- Relate to Everyday Examples: Connect atomic structure to real-world examples, like the properties of different materials or the chemical reactions involved in cooking.
- Use Resources: Utilize textbooks, online resources, and educational videos to deepen your understanding.
Conclusion
The atom, with its intricate and fascinating structure, lies at the heart of our understanding of matter and the universe. Its fundamental components, the nucleus and electron cloud, govern the properties of elements and determine how they interact to form molecules and complex materials. Understanding atomic structure is crucial for unraveling the mysteries of chemistry, physics, and material science, paving the way for advancements in technology and our knowledge of the universe. As we continue to explore the realm of the atom, we gain deeper insights into the fundamental building blocks of our reality, unlocking the secrets of the universe one particle at a time.







Closure
Thus, we hope this article has provided valuable insights into Unveiling the Building Blocks of Matter: A Deep Dive into the Atomic Structure. We appreciate your attention to our article. See you in our next article!
